

Lower-energy conformations result from having nonbonding electron pairs in equatorial, rather than axial, positions in this geometry. There are two distinct positions in this geometry: Axial Equatorial 18 16ġ7 Exercise 9.1 1) Use the VSEPR model to predict the molecular geometry of A) O3 B) SnCl3- 2) Predict the electron-domain geometry and the molecular geometry for A) SeCl2 B) CO32- 3) Draw the structure for NO3- and describe the bond angles. There are three molecular geometries: Tetrahedral, if all are bonding pairs, Trigonal pyramidal if one is a nonbonding pair, Bent if there are two nonbonding pairs. 14ĭouble and triple bonds place greater electron density on one side of the central atom than do single bonds. Therefore, their repulsions are greater this tends to decrease bond angles in a molecule. Nonbonding pairs are physically larger than bonding pairs. There are two molecular geometries: Trigonal planar, if all the electron domains are bonding, Bent, if one of the domains is a nonbonding pair. NOTE: If there are only two atoms in the molecule, the molecule will be linear no matter what the electron domain is. In the linear domain, there is only one molecular geometry: linear. 10ġ1 Molecular Geometries Within each electron domain, then, there might be more than one molecular geometry. The molecular geometry is that defined by the positions of only the atoms in the molecules, not the nonbonding pairs. 9ġ0 Molecular Geometries The electron-domain geometry is often not the shape of the molecule, however. The geometry will be that which corresponds to the number of electron domains. Which shape could lead to square planar geometry upon removal of one or more atoms? 8Īll one must do is count the number of electron domains in the Lewis structure.
All 5 atoms lie in the same plane with the B atoms on the corner of a square and A in the middle.

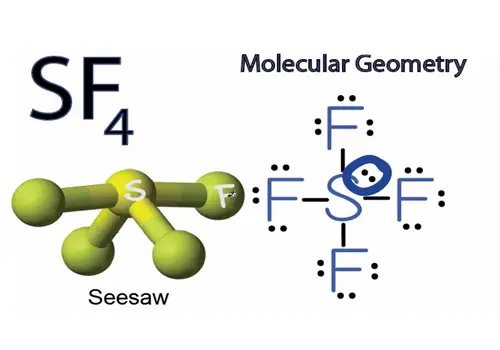
P.344 GIST: One of the common shapes for AB4 molecules is square planar. “The best arrangement of a given number of electron domains is the one that minimizes the repulsions among them.” 7 5ħ Valence Shell Electron Pair Repulsion Theory (VSEPR) The central atom in this molecule, A, has four electron domains. In a double or triple bond, all electrons shared between those two atoms are on the same side of the central atom therefore, they count as one electron domain.

4ĥ Electron Domains We can refer to the electron pairs as electron domains. By assuming the electron pairs are placed as far as possible from each other, we can predict the shape of the molecule. Simply put, electron pairs, whether they be bonding or nonbonding, repel each other. 3Ĥ What Determines the Shape of a Molecule? By noting the number of bonding and nonbonding electron pairs we can easily predict the shape of the molecule. 1 Chapter 9 Molecular Geometry and Bonding Theoriesģ Molecular Shapes The shape of a molecule plays an important role in its reactivity.


 0 kommentar(er)
0 kommentar(er)
